Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
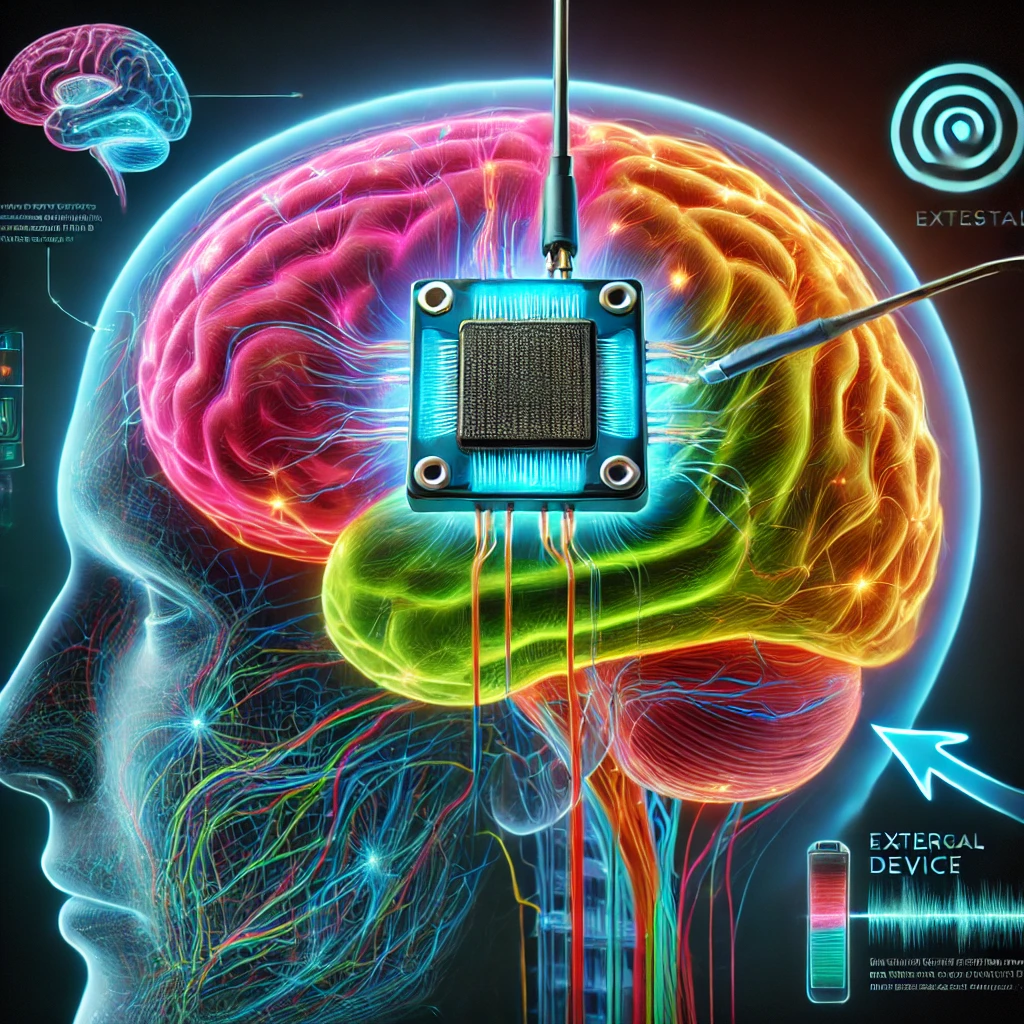
Introduction:
Neuralink, the brain-machine interface company co-founded by Elon Musk, is positioning itself at the forefront of one of the most ambitious technological advancements in history: enabling humans to control devices with their minds. The company’s goal is nothing short of revolutionary, aiming to create seamless communication between the brain and external devices like computers, prosthetics, and even robots. Neuralink’s technology could drastically improve the lives of people with neurological conditions, giving them the ability to regain control over their environment in ways never before possible. However, despite these promising advancements, the company is also facing increasing scrutiny and regulatory hurdles. In this post, we’ll take a closer look at the latest developments, including their first successful human trial, the ongoing controversy over animal testing, and the future of this groundbreaking technology.
The First Human Trial: Mind-Controlled Technology
In a monumental leap forward for Neuralink, the company has successfully conducted a human trial where a participant was able to control a computer mouse entirely through thought. This trial represents a significant milestone in the development of brain-machine interfaces (BMIs), providing concrete proof that it’s possible to translate brain signals into real-world actions. The process is accomplished by implanting a small chip into the brain, which then communicates wirelessly with external devices. The chip, which is smaller than a coin, is designed to read the brain’s neural signals and translate them into commands that control various devices like computers or robotic arms.
For people with severe disabilities, especially those suffering from paralysis, this technology could open new doors. Imagine a person who has lost the ability to move their limbs being able to control a wheelchair, a computer, or even a prosthetic limb, all with the power of their thoughts. The potential for improving the quality of life for those with neurological conditions is immense. Neuralink has already begun working on these possibilities, and this trial serves as just the beginning of what could become a transformative medical tool. As the technology advances, it’s expected that more complex tasks, such as controlling entire systems or even restoring motor function, could become feasible.
Ethical Concerns: FDA Citation on Animal Testing
While Neuralink’s human trials show promise, the company has faced significant backlash in another area: its animal testing practices. Recently, the U.S. Food and Drug Administration (FDA) cited Neuralink for “objectionable conditions” in its animal lab. The citation highlights concerns about inadequate care for animals and insufficient documentation related to the testing process. These concerns are particularly troubling given the scale of the animal trials, which have involved the implantation of neural chips in monkeys and other animals to test the technology’s safety and efficacy.
The ethical debate surrounding animal testing in such advanced technologies is complex. On one hand, the testing is necessary to ensure the safety and effectiveness of neural implants before they can be tested on humans. On the other hand, the use of animals in experiments that can involve invasive surgeries and potential harm raises significant moral questions. Critics argue that companies like Neuralink should be held to higher standards when it comes to the treatment of animals, especially given the cutting-edge nature of the technology they’re developing.
Despite the citation, Neuralink has responded by claiming they are committed to improving the conditions in their lab and addressing the FDA’s concerns. The company has made efforts to comply with regulatory standards and is working on improving their protocols. However, the ethical and regulatory hurdles surrounding animal testing remain a significant challenge for the company, potentially delaying the approval of their technology and raising public skepticism.
The Road Ahead: Opportunities and Challenges
Looking ahead, Neuralink has the potential to revolutionize healthcare and human-computer interaction. The possibilities for using brain-machine interfaces are virtually limitless. Beyond restoring mobility for people with disabilities, Neuralink could also enable a host of other advancements, such as direct brain-to-brain communication, enhanced cognitive abilities, and even new treatments for conditions like Alzheimer’s, Parkinson’s, and traumatic brain injuries. For those suffering from diseases that affect the brain, Neuralink’s technology could offer hope for new treatments that might not have been possible with traditional medical methods.
However, the road to widespread use of Neuralink’s technology is filled with obstacles. From a regulatory standpoint, the FDA’s concerns regarding animal testing and the safety of the implants will need to be addressed before the technology can be approved for large-scale human use. The company will also need to navigate public concerns about privacy and the potential misuse of brain-computer interfaces. There’s also the question of long-term safety—implanting a chip in the brain carries risks, and we still don’t know the full impact of these implants on brain function or whether there could be unforeseen side effects over time.
Despite these challenges, Neuralink’s success in the human trial is a significant step forward. The company has shown that the technology works, and the potential benefits for humanity are immense. But it remains to be seen whether Neuralink can overcome the ethical, regulatory, and safety concerns that stand in its way.
Conclusion:
Neuralink stands at a critical juncture. With groundbreaking advancements in brain-machine interfaces and the ability to control technology with nothing more than thought, the company is poised to change the future of healthcare and human-computer interaction. But as we’ve seen, the road ahead is filled with challenges. From ethical concerns over animal testing to regulatory hurdles and the risks associated with brain implants, Neuralink must address these issues if it’s to succeed in bringing this revolutionary technology to the public. Whether Neuralink will achieve its goal of enhancing human capabilities and improving lives, or if it will falter due to these controversies, remains to be seen. One thing is certain: Neuralink is pushing the boundaries of what’s possible, and the future of brain-machine interfaces will likely be shaped by its success or failure.